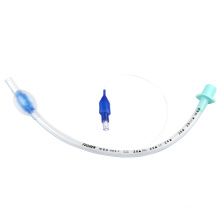
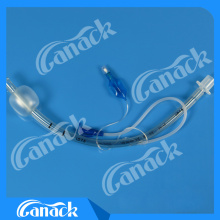
Ce and ISO Approved Standard Endotracheal Tube
Ce and ISO Approved Standard Endotracheal Tube
| Payment Type: | L/C, T/T, Western Union, Paypal |
|---|---|
| Min. Order: | 1000 |
| Productivity: | 50000 PCS/Month |
|---|---|
| Place of Origin: | China |
| Supply Ability: | 50000 PCS/Month |
| Certificate: | CE, ISO13485 |
Basic Info
Model No.: ET
Product Description
Model NO.: ET Material: Medical Grade PVC Material Ethylene Oxide Sterilization: Ethylene Oxide Sterilization Group: Adult, Children, Infant Medical Device Regulatory Type: Type 2 Cuff: Cuff/Uncuff OEM/ODM: Welcome Transport Package: Plastic Paper Pouch or Hard Blister Package Origin: China Type: Surgical Supplies Materials Certification: CE, ISO13485 Quality Guarantee Period: Five Years Logo Printing: Canack or OEM Color: Transparent Free Sample: Yes Trademark: Canack or OEM Specification: 3.0mm-9.5mm Reinforced Endotracheal Tube
1.Non-toxic, medical grade PVC material.
2.X-ray opaque line.
3.Clear markings for easy observation.
4.High volume low pressure cuff maintains good sealing.
5.Murphy eye design.
6.Smooth cuff edges reduce trauma.
Packaging & Shipping
Packaging Details: Plastic-paper packing or blister package,sterilized pouch.
Delivery Detail: about 25 days after receipt of deposit
Our service
1.Your inquiry related to our products or prices will be replied in 24hrs.
2.We have well-trained and experienced staffs to co-operation with you.
3. Protection of your sales area, ideas of design and all your private information.
Company Information
Canack Technology Ltd. is an international trading company specialized in consumable medical devices, registered in the BC Canada since 2004.
With the rapid development, we have invested and established our own medical device factory and a trading company (Hangzhou Nanaimo Trading Co., Ltd.) in China which specialize in the development, manufacturing and distribution of Disposable Medical products.
By now, our product lines cover the area of Anesthesia, Respiratory and Wound Drains. The main products include Laryngeal Mask Airway, Silicone Drainage Tube, Wound Drainage System, Breathing Circuit and Breathing Filters etc.
All our products are designed and manufactured to meet the requirements of relevant ISO13485 standards, CE certified.
We Canack staffs take care of patients' health with quality products; we believe this core values can help us grow and serve the society better.
FAQ
1.Q:Are you a factory or trading company?A: We are a factory, we have rich experience for manufacturing more than 10 years.
2.Q:Where is your factory located? How can i visit there?
A: Our factory is located in Yuyao city,Zhejiang Province,China.It is about more than 3 hours from Shanghai airport, We can pick you up if you need, welcome to visit our factory.
3.Q:What is the material for your products?
A. The material is silicone.
4.Q:Can you make samples?
A: Yes, we can make samples for you.
5.Q:How does your factory do as quality control?A: Quality is the lift of our factory. We have inspector in different post, and there are three inspectors before packing to get best quality products.
Contact us if you need more details on Endotracheal tube. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Medical Consumables、Tube. If these products fail to match your need, please contact us and we would like to provide relevant information.
1.Non-toxic, medical grade PVC material.
2.X-ray opaque line.
3.Clear markings for easy observation.
4.High volume low pressure cuff maintains good sealing.
5.Murphy eye design.
6.Smooth cuff edges reduce trauma.
| Cat. No. | Size ID (mm) | Cat. No. | Size ID (mm) |
| ET30PC | 3.0 | ET65PC | 6.5 |
| ET35PC | 3.5 | ET70PC | 7.0 |
| ET40PC | 4.0 | ET75PC | 7.5 |
| ET45PC | 4.5 | ET80PC | 8.0 |
| ET50PC | 5.0 | ET85PC | 8.5 |
| ET55PC | 5.5 | ET90PC | 9.0 |
| ET60PC | 6.0 | ET95PC | 9.5 |
Packaging & Shipping
Packaging Details: Plastic-paper packing or blister package,sterilized pouch.
Delivery Detail: about 25 days after receipt of deposit
Our service
1.Your inquiry related to our products or prices will be replied in 24hrs.
2.We have well-trained and experienced staffs to co-operation with you.
3. Protection of your sales area, ideas of design and all your private information.
Company Information
Canack Technology Ltd. is an international trading company specialized in consumable medical devices, registered in the BC Canada since 2004.
With the rapid development, we have invested and established our own medical device factory and a trading company (Hangzhou Nanaimo Trading Co., Ltd.) in China which specialize in the development, manufacturing and distribution of Disposable Medical products.
By now, our product lines cover the area of Anesthesia, Respiratory and Wound Drains. The main products include Laryngeal Mask Airway, Silicone Drainage Tube, Wound Drainage System, Breathing Circuit and Breathing Filters etc.
All our products are designed and manufactured to meet the requirements of relevant ISO13485 standards, CE certified.
We Canack staffs take care of patients' health with quality products; we believe this core values can help us grow and serve the society better.
FAQ
1.Q:Are you a factory or trading company?A: We are a factory, we have rich experience for manufacturing more than 10 years.
2.Q:Where is your factory located? How can i visit there?
A: Our factory is located in Yuyao city,Zhejiang Province,China.It is about more than 3 hours from Shanghai airport, We can pick you up if you need, welcome to visit our factory.
3.Q:What is the material for your products?
A. The material is silicone.
4.Q:Can you make samples?
A: Yes, we can make samples for you.
5.Q:How does your factory do as quality control?A: Quality is the lift of our factory. We have inspector in different post, and there are three inspectors before packing to get best quality products.
Contact us if you need more details on Endotracheal tube. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Medical Consumables、Tube. If these products fail to match your need, please contact us and we would like to provide relevant information.
Product Categories : Anesthsia Series > Endotracheal Tube
Premium Related Products
Other Products
Hot Products
Disposable Infusion PumpAnimal Medical Silicone Round Channel /Fluted DrainAnimal Products Disposable Silicone Reservoir 100ml/150ml/200ml/400mlDisposable Epidural-Spinal Combined Anesthesia KitPVC Manual ResuscitatorLatex Foley CatheterHollow Closed Wound Drainage SystemDisposable Medical Drainage BagCe/ISO 13485 Medical Silicone Anesthesia Breathing CircuitSurgical Products Breathing Circuit -Corrugated for AdultMedical Product Standard Endotracheal TubeSurgical Sterile Disposable Hmef FilterDisposable Medical Consumables PVC Anesthesia Mask with Check ValveHigh Quality Color-Coded Oral Pharyngeal Guedel Airway Made in ChinaMedical Equipment Face Mask Oxygen Mask with TubeCe & ISO Approved Disposable Silicone Laryngeal Mask Airway