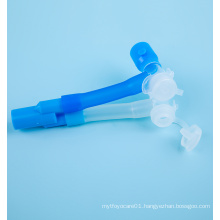
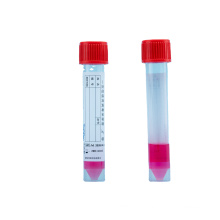
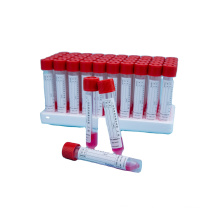
Medical one-time Inactivated Virus Sampling Tube
Medical one-time Inactivated Virus Sampling Tube
| Min. Order: | 10000 Piece/Pieces |
|---|
| Packaging: | export standard |
|---|---|
| Productivity: | 10 000 000 pcs/month |
| Brand: | Uni-medica |
| Transportation: | Ocean,Land,Air,Express |
| Place of Origin: | China |
| Supply Ability: | 10 000 000 000 pcs/year |
| Certificate: | CE/SGS |
| HS Code: | 3821000000 |
| Port: | Shenzhen |
Basic Info
Model No.: VST-02
Click on the follow link to find out more information: https://www.unimed-global.com/inactivated-disposable-virus-sampling-tube/
Company Info
- Company Name: Shenzhen Uni-medica Technology Co.,Ltd
- Representative: Wang yan Pin
- Product/Service: Total solution of SARS-CoV-2 , RT-PCR test kits , Multiplex Fluorescence Staining Solution , Pathogens targeted NGS , Newborn Genetic Screening NGS , Immunochromatography POCT
- Capital (Million US $): 5,000,000RMB
- Year Established: 2011
- Total Annual Sales Volume (Million US $): US$10 Million - US$50 Million
- Export Percentage: 51% - 60%
- Total Annual Purchase Volume (Million US $): US$5 Million - US$10 Million
- No. of Production Lines: 5
- No. of R&D Staff: 71 -80 People
- No. of QC Staff: 51 -60 People
- OEM Services Provided: yes
- Factory Size (Sq.meters): 1,000-3,000 square meters
- Factory Location: 2nd Floor, No.6 Building, Xili Liuxian Culture Park, Nanshan District, Shenzhen City, Guangdong Province, China
- Contact Person: Ms. Super D~D
- Tel: +86-755-86505501
Premium Related Products
Other Products
Hot Products
Disposable Infusion PumpAnimal Medical Silicone Round Channel /Fluted DrainAnimal Products Disposable Silicone Reservoir 100ml/150ml/200ml/400mlDisposable Epidural-Spinal Combined Anesthesia KitPVC Manual ResuscitatorLatex Foley CatheterHollow Closed Wound Drainage SystemDisposable Medical Drainage BagCe/ISO 13485 Medical Silicone Anesthesia Breathing CircuitSurgical Products Breathing Circuit -Corrugated for AdultMedical Product Standard Endotracheal TubeSurgical Sterile Disposable Hmef FilterDisposable Medical Consumables PVC Anesthesia Mask with Check ValveHigh Quality Color-Coded Oral Pharyngeal Guedel Airway Made in ChinaMedical Equipment Face Mask Oxygen Mask with TubeCe & ISO Approved Disposable Silicone Laryngeal Mask Airway